

All beginners, experienced and university/campus/collage/school students. State Kelvin Planck and Clausius statement and establish the equivalence of both for second law of thermodynamics. – Anyone preparing for entrance examinations and other competitive examinations.
#KELVIN PLANCK STATEMENT OF SECOND LAW OF THERMODYNAMICS CRACK#
– students who want to crack any examination related to Thermodynamics. 1.4, it is impossible to construct a device that, operating in a cycle, will produce no effect other than the transfer of heat from a cold body to a hot one. – students who want to test thiers eligibility in Thermodynamics. The second law of thermodynamics can also be expressed in terms of the Clausius and Kelvin-Planck statements: Clausius statement: As shown in Fig. – Students who wish to gain extra knowledge about Thermodynamics. – Students who are preparing for examinations such as Class Tests, Mid-term tests end-terms tests and semester tests on Thermodynamics. – anyone who are Preparing For Online/Offline Tests In Thermodynamics.
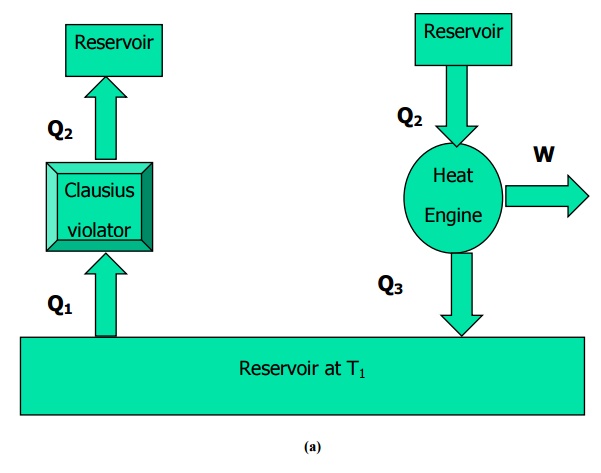
Our Thermodynamics Interview questions come with detailed explanation of the answers which makes better understanding of Thermodynamics concepts. Depending on the type of engine, the processes that the working fluid undergo will be different, but they will always constitute a clockwise thermodynamic cycle. The concept of reversibility, Carnot cycle and Carnot principle is introduced. The idea of a machine with 100 thermal efficiency is rejected. The Kelvin Planck statement and its corollary - the Clausius Statement is discussed. They can be a beginner, fresher, engineering graduate or an experienced IT professional. This chapter discusses the limitations of first law and introduces the second law of thermodynamics. These Multiple choice questions and Answers can be attempted by anyone who focusing on learning Thermodynamics. it is impossible to construct an engine working on a cyclic process, whose sole. Regular practice these multiple choice questions and answers(mCQ) to improve their Thermodynamics skills which help you to crack Entrance Exams, Competitive Exams, campus interviews, company interviews And placements. According to Kelvin-Plancks statement of second law of thermodynamics. It states that: It is impossible for any device that operates on a cycle. This section focuses on “Second Law of Thermodynamics”. The Kelvin-Planck Statement is another expression of the second law of thermodynamics. KelvinPlanck statement Clausius statement. 100+ MCQS on Second Law of Thermodynamics. The first law of thermodynamics states that during any cycle that a system undergoes, the cyclic integral.


 0 kommentar(er)
0 kommentar(er)
